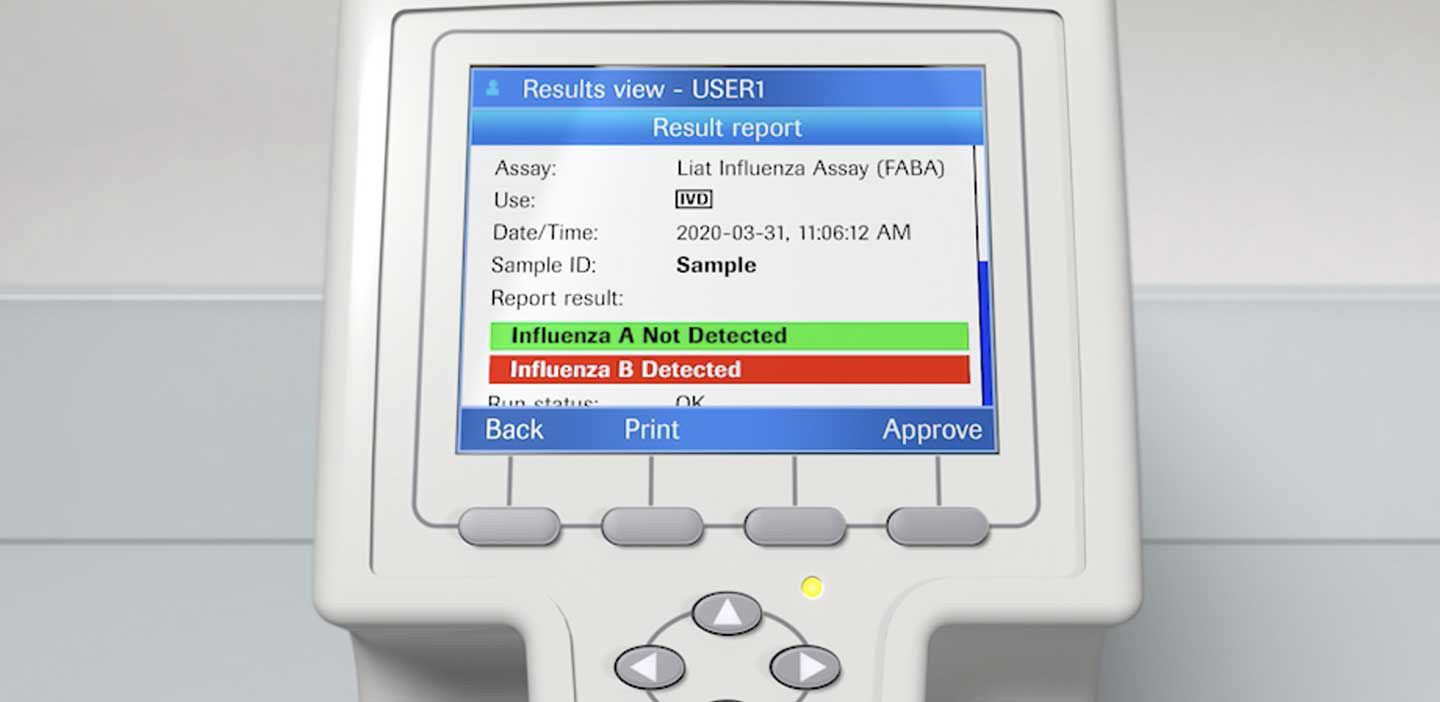
cobas SARS-CoV-2 & Influenza A/B Nucleic Acid Test for use on the cobas Liat System - Healthcare Provider Fact Sheet

FDA authorized molecular point-of-care SARS-CoV-2 tests: A critical review on principles, systems and clinical performances - ScienceDirect

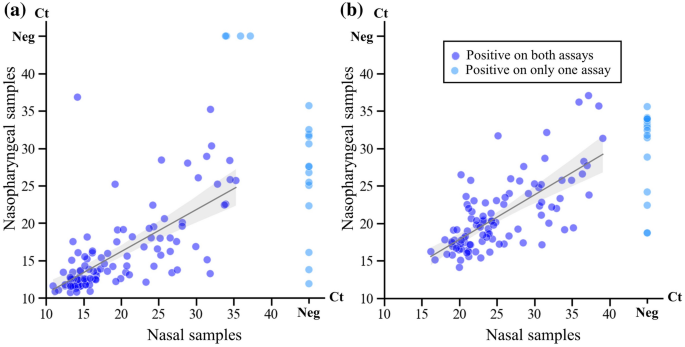
Performance Characteristics of the Roche Diagnostics cobas Liat PCR System as a COVID-19 Screening Tool for Hospital Admissions in a Regional Health Care Delivery System | Journal of Clinical Microbiology
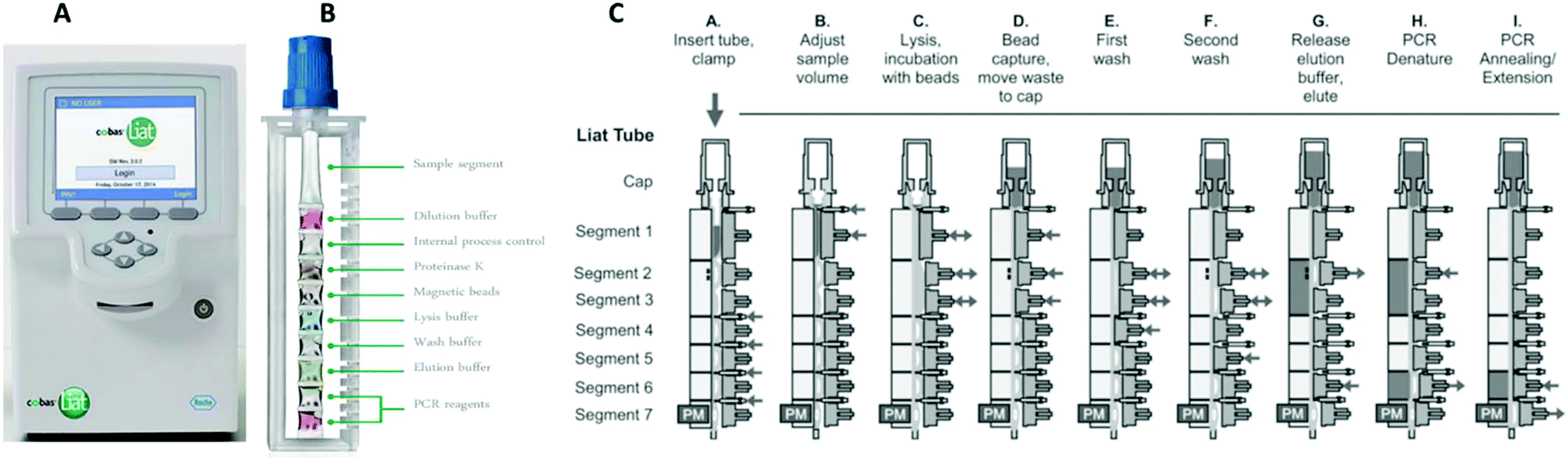
Current state of commercial point-of-care nucleic acid tests for infectious diseases - Analyst (RSC Publishing) DOI:10.1039/D0AN01988G
Quick Reference Instructions cobas® SARS-CoV-2 & Influenza A/B Nucleic acid test for use on the cobas®Liat® System For u

Roche gives lab professionals firsthand glimpse of connected lab of the future at AACC 2016 Clinical Lab Expo