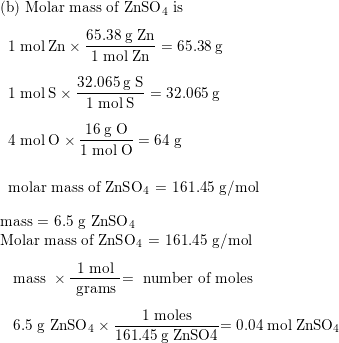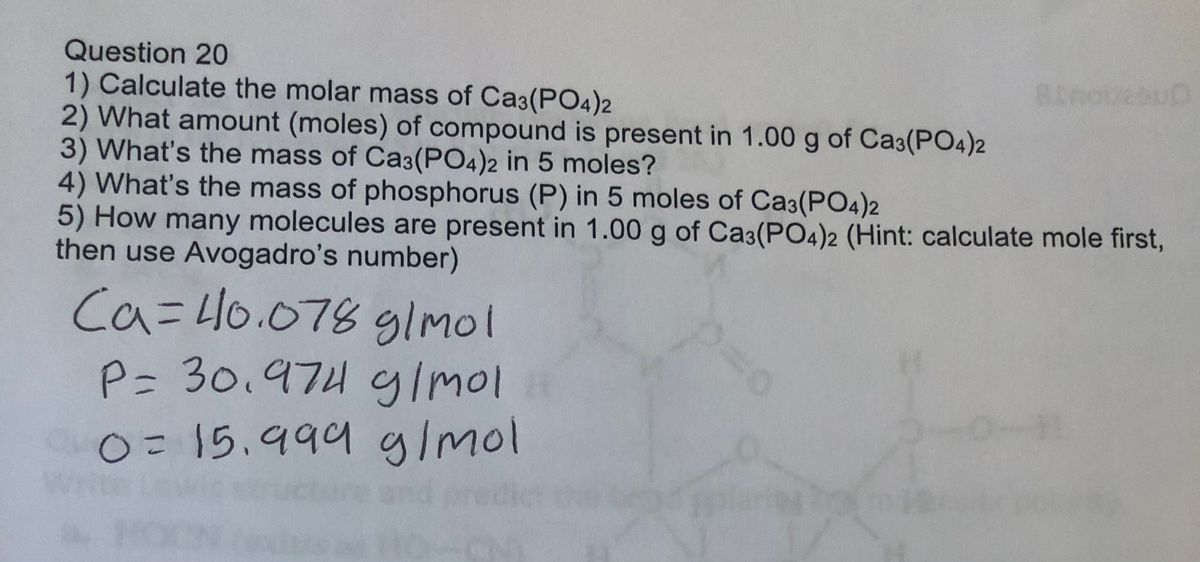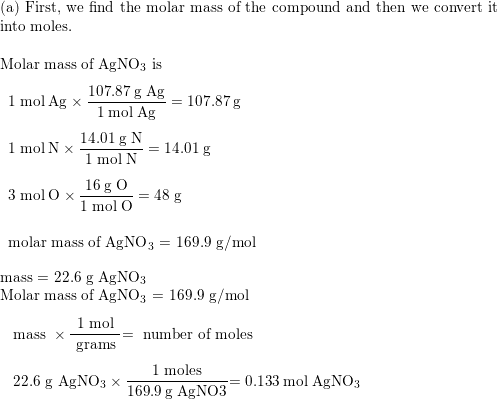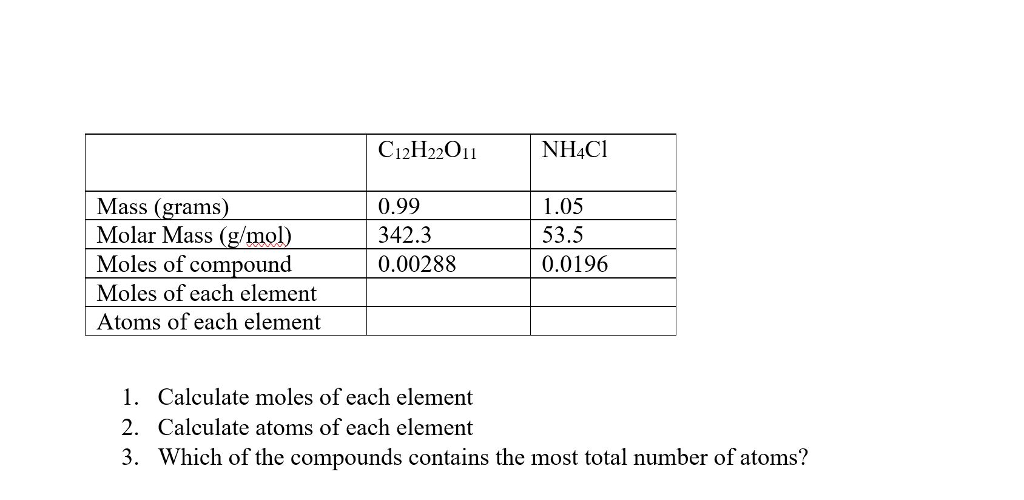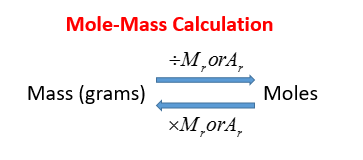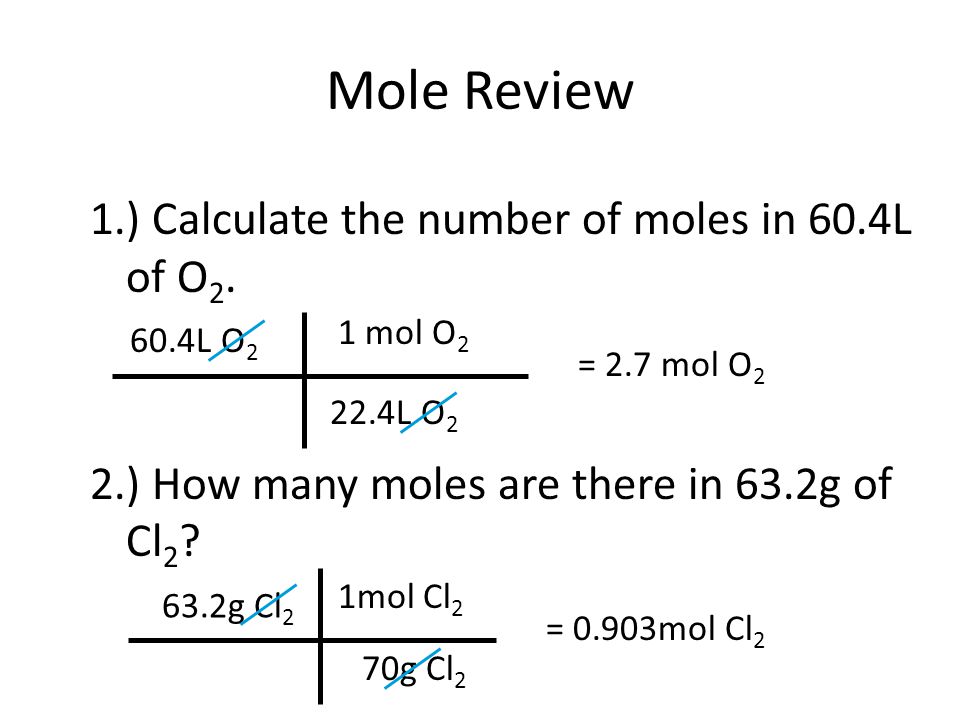
Mole Review 1.) Calculate the number of moles in 60.4L of O2. 2.) How many moles are there in 63.2g of Cl2? 1 mol O2 60.4L O2 = 2.7 mol O2 22.4L

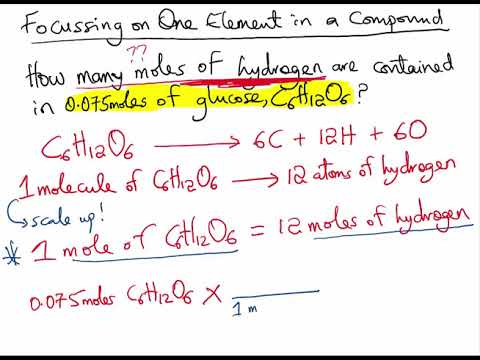
If 3 moles of ethane (C2H6) are given, calculate the following:(i) Number of moles of carbon atoms. (ii) Number of moles of hydrogen atoms. (iii) Number of molecules of ethane.

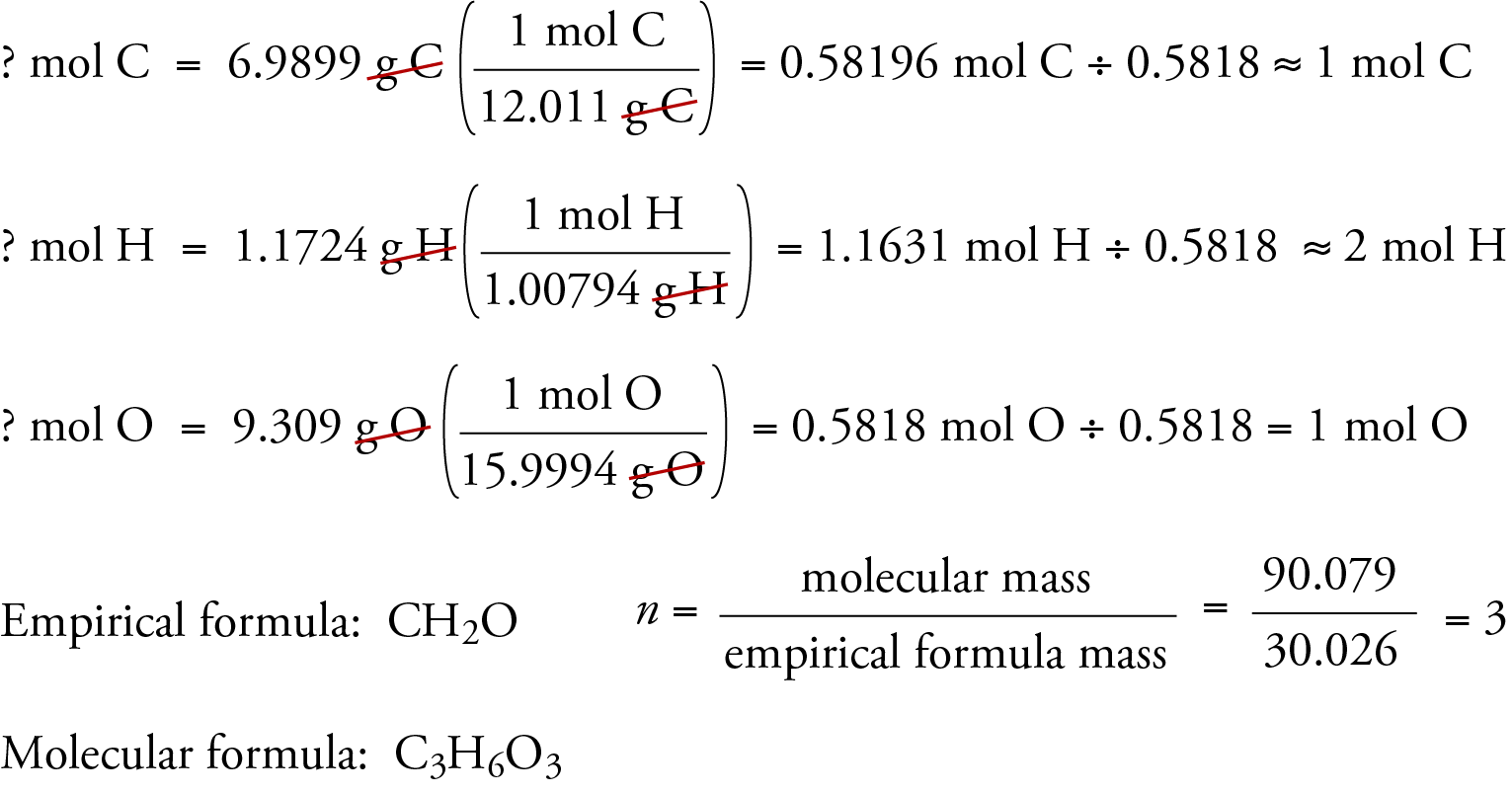
How do I find the ratios between the number of moles of each element to determine the formula of the compound? | Socratic

stoichiometry//moles: i understand you're looking for number or moles for hydrogen, but why do u multiply the number of hydrogen atoms by the amount of molecules in the compound?? i don't get

SOLVED: The chemical formula for chlorophyll is C₅₅H₇₂MgN₄O₅. Given the molar mass of the compound is 893.49 g/mol, what is the mass in grams of chlorophyll that are in 5.959 moles of


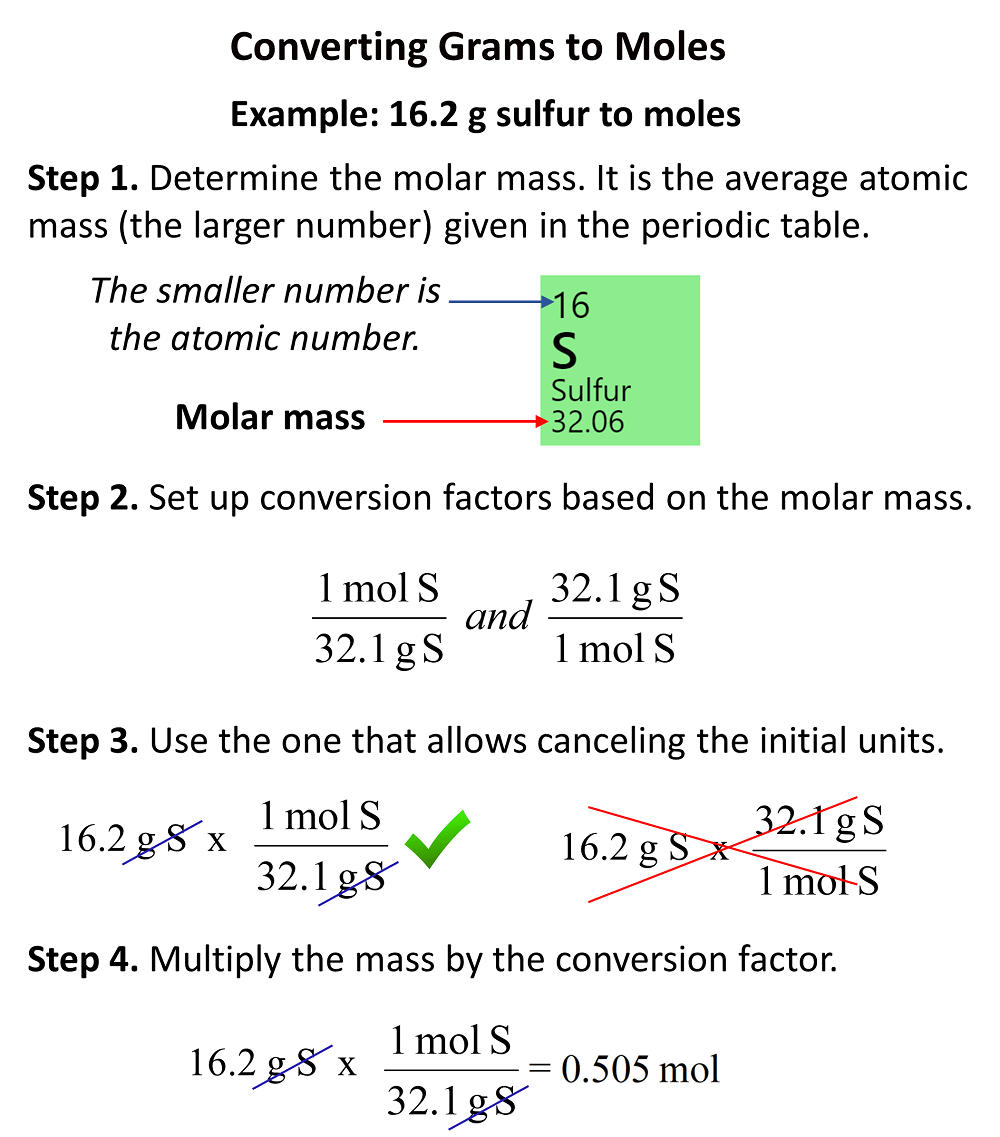
![Example] How to Calculate Moles From the Mass of a Compound - Converting Mass to Moles. - YouTube Example] How to Calculate Moles From the Mass of a Compound - Converting Mass to Moles. - YouTube](https://i.ytimg.com/vi/EKywe4xU4cs/maxresdefault.jpg)