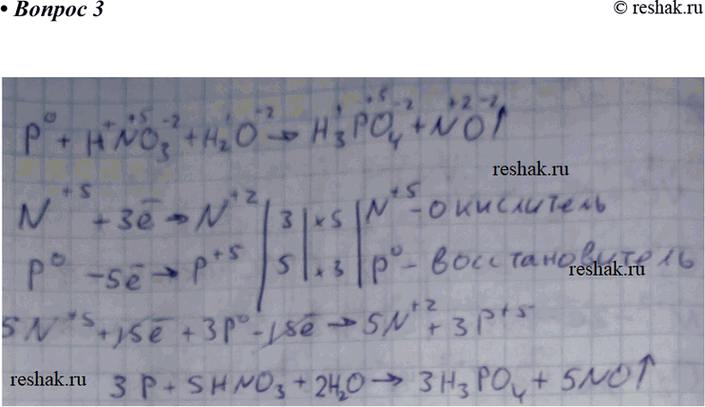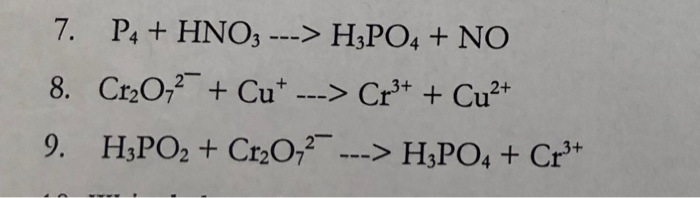Balance the following equation by partial equation method: P4 + HNO3 = H3PO4 + NO2 + H2O | Homework.Study.com

Balance the following equations a Fe H2O Fe3O4 H2 b Ca N2 Ca3N2 c Zn KOH K2ZnO2 H2 d Fe2O3 CO Fe CO2...

HNO3/H3PO4–NANO2 mediated oxidation of cellulose — preparation and characterization of bioabsorbable oxidized celluloses in high yields and with different levels of oxidation - ScienceDirect

Oxidation Number method. P4+HNO3+H2O=H3PO4+NO. Balance the equation by oxidation Number method. - YouTube